🧠 Hands-On Medical Image Classification: Best Practices & Common Pitfalls#
📋 Table of Contents#
Introduction & Environment Setup#
In this comprehensive tutorial, you’ll build a medical image classifier using real-world best practices that are crucial for healthcare applications. We’ll work with a chest X-ray dataset and highlight common mistakes that can compromise model performance and clinical applicability.
Learning Objectives:#
How to properly handle medical image data with its unique challenges
How to prevent data leakage that can severely impact real-world performance
How to leverage transfer learning effectively with limited medical datasets
How to apply advanced validation strategies specific to medical imaging
How to address class imbalance typical in medical datasets

Environment Setup#
# Basic libraries
import os
import random
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
from matplotlib.colors import ListedColormap
from PIL import Image
import warnings
warnings.filterwarnings('ignore')
# PyTorch
import torch
import torchvision
from torchvision import transforms, models
from torch.utils.data import DataLoader, Dataset, WeightedRandomSampler, random_split
import torch.nn as nn
import torch.nn.functional as F
import torch.optim as optim
from torch.optim.lr_scheduler import ReduceLROnPlateau
# Metrics and evaluation
from sklearn.metrics import (
classification_report, confusion_matrix,
roc_auc_score, roc_curve, precision_recall_curve,
accuracy_score, f1_score, precision_score, recall_score
)
from sklearn.model_selection import KFold, StratifiedKFold
import seaborn as sns
# Set seeds for reproducibility
def set_seed(seed=42):
random.seed(seed)
np.random.seed(seed)
torch.manual_seed(seed)
torch.cuda.manual_seed(seed)
torch.backends.cudnn.deterministic = True
torch.backends.cudnn.benchmark = False
set_seed()
# Check if GPU is available
device = torch.device("cuda" if torch.cuda.is_available() else "cpu")
print(f"Using device: {device}")
---------------------------------------------------------------------------
ModuleNotFoundError Traceback (most recent call last)
Cell In[1], line 5
3 import random
4 import numpy as np
----> 5 import pandas as pd
6 import matplotlib.pyplot as plt
7 from matplotlib.colors import ListedColormap
ModuleNotFoundError: No module named 'pandas'
Dataset Information#
This tutorial uses the Chest X-ray Pneumonia dataset from Kaggle, which contains:
Normal chest X-rays from healthy patients
Chest X-rays with pneumonia (including bacterial and viral cases)
The dataset has clinical significance as pneumonia is a leading cause of mortality, especially in children under 5 years old. Computer-aided diagnosis can help in resource-limited settings where radiologists may not be readily available.
Dataset Loading and Inspection#
Medical image datasets often have specific directory structures and metadata. Let’s learn how to properly load and examine them.

# Dataset path structure:
# chest_xray/
# train/NORMAL/
# train/PNEUMONIA/
# val/...
# test/...
data_dir = "/kaggle/input/chest-xray-pneumonia/chest_xray"
# Define transformations with medical imaging best practices
train_transform = transforms.Compose([
transforms.Resize((128, 128)),
transforms.RandomHorizontalFlip(), # Data augmentation
transforms.RandomRotation(10), # Slight rotation
transforms.ToTensor(),
# Normalize to ImageNet stats - standard practice for transfer learning
transforms.Normalize(mean=[0.485, 0.456, 0.406], std=[0.229, 0.224, 0.225])
])
# Validation and test transforms should NOT include augmentation
val_test_transform = transforms.Compose([
transforms.Resize((128, 128)),
transforms.ToTensor(),
transforms.Normalize(mean=[0.485, 0.456, 0.406], std=[0.229, 0.224, 0.225])
])
Creating Dataset Objects#
from torchvision.datasets import ImageFolder
# Load datasets with appropriate transforms
train_dataset = ImageFolder(os.path.join(data_dir, "train"), transform=train_transform)
val_dataset = ImageFolder(os.path.join(data_dir, "val"), transform=val_test_transform)
test_dataset = ImageFolder(os.path.join(data_dir, "test"), transform=val_test_transform)
# Examine dataset statistics
print(f"Classes: {train_dataset.classes}")
print(f"Class indices: {train_dataset.class_to_idx}")
print(f"Train samples: {len(train_dataset)}")
print(f"Validation samples: {len(val_dataset)}")
print(f"Test samples: {len(test_dataset)}")
# Count samples per class
def count_samples_per_class(dataset):
counts = {}
for _, idx in dataset.samples:
label = dataset.classes[idx]
counts[label] = counts.get(label, 0) + 1
return counts
train_counts = count_samples_per_class(train_dataset)
val_counts = count_samples_per_class(val_dataset)
test_counts = count_samples_per_class(test_dataset)
print("\nClass distribution:")
print(f"Train: {train_counts}")
print(f"Validation: {val_counts}")
print(f"Test: {test_counts}")
Classes: ['NORMAL', 'PNEUMONIA']
Class indices: {'NORMAL': 0, 'PNEUMONIA': 1}
Train samples: 5216
Validation samples: 16
Test samples: 624
Class distribution:
Train: {'NORMAL': 1341, 'PNEUMONIA': 3875}
Validation: {'NORMAL': 8, 'PNEUMONIA': 8}
Test: {'NORMAL': 234, 'PNEUMONIA': 390}
Custom Dataset for Advanced Preprocessing#
class ChestXrayDataset(Dataset):
"""Custom dataset that includes image file paths and patient IDs"""
def __init__(self, image_folder, transform=None):
self.image_folder = image_folder
self.transform = transform
self.classes = ["NORMAL", "PNEUMONIA"]
self.class_to_idx = {"NORMAL": 0, "PNEUMONIA": 1}
# Get all image paths and their corresponding labels
self.samples = []
for class_name in self.classes:
class_dir = os.path.join(image_folder, class_name)
if not os.path.isdir(class_dir):
continue
for img_name in os.listdir(class_dir):
if img_name.endswith(('.jpeg', '.jpg', '.png')):
# Extract patient ID from filename (e.g., "patient1234_1.jpg" -> "patient1234")
patient_id = img_name.split('_')[0] if '_' in img_name else img_name.split('.')[0]
img_path = os.path.join(class_dir, img_name)
label = self.class_to_idx[class_name]
self.samples.append((img_path, label, patient_id))
def __len__(self):
return len(self.samples[0:100])
def __getitem__(self, idx):
img_path, label, patient_id = self.samples[idx]
# Load and transform image
image = Image.open(img_path).convert('RGB')
if self.transform:
image = self.transform(image)
return image, label, patient_id
Exploratory Data Analysis & Preprocessing#
Understanding your dataset is crucial before building any model. Let’s examine the images and their characteristics.
# Function to visualize sample images
def show_samples(dataset, n=5):
fig, axs = plt.subplots(1, n, figsize=(15, 3))
# Get random indices
indices = random.sample(range(len(dataset)), n)
for i, idx in enumerate(indices):
img, label = dataset[idx]
# Convert tensor to numpy for display
img_np = img.numpy().transpose((1, 2, 0))
# De-normalize for visualization
mean = np.array([0.485, 0.456, 0.406])
std = np.array([0.229, 0.224, 0.225])
img_np = std * img_np + mean
img_np = np.clip(img_np, 0, 1)
# Display image
axs[i].imshow(img_np)
axs[i].set_title(f"{dataset.classes[label]}")
axs[i].axis('off')
plt.tight_layout()
plt.show()
# Show sample images
print("Sample training images:")
show_samples(train_dataset)
Sample training images:
Analyzing Image Properties#
# Function to analyze image properties
def analyze_image_properties(dataset, n=100):
widths, heights, aspect_ratios = [], [], []
# Sample images
indices = random.sample(range(len(dataset)), min(n, len(dataset)))
for idx in indices:
img_path, _ = dataset.samples[idx]
with Image.open(img_path) as img:
w, h = img.size
widths.append(w)
heights.append(h)
aspect_ratios.append(w/h)
# Plot histograms
fig, axs = plt.subplots(1, 3, figsize=(15, 4))
axs[0].hist(widths, bins=20)
axs[0].set_title('Width Distribution')
axs[0].set_xlabel('Width (pixels)')
axs[1].hist(heights, bins=20)
axs[1].set_title('Height Distribution')
axs[1].set_xlabel('Height (pixels)')
axs[2].hist(aspect_ratios, bins=20)
axs[2].set_title('Aspect Ratio Distribution')
axs[2].set_xlabel('Width/Height')
plt.tight_layout()
plt.show()
# Print statistics
print(f"Width - Min: {min(widths)}, Max: {max(widths)}, Mean: {np.mean(widths):.1f}")
print(f"Height - Min: {min(heights)}, Max: {max(heights)}, Mean: {np.mean(heights):.1f}")
print(f"Aspect Ratio - Min: {min(aspect_ratios):.2f}, Max: {max(aspect_ratios):.2f}, Mean: {np.mean(aspect_ratios):.2f}")
# Analyze images
analyze_image_properties(train_dataset)
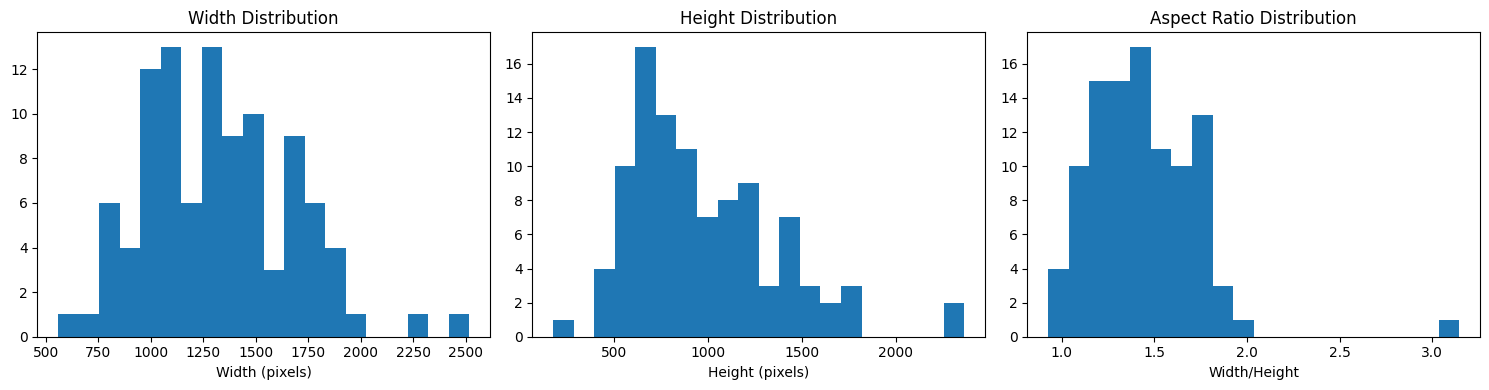
Width - Min: 557, Max: 2518, Mean: 1324.0
Height - Min: 177, Max: 2364, Mean: 977.2
Aspect Ratio - Min: 0.93, Max: 3.15, Mean: 1.44
Analyzing Pixel Intensity Distribution#
def analyze_pixel_distribution(dataset, n=20):
pixel_values = []
# Sample images
indices = random.sample(range(len(dataset)), min(n, len(dataset)))
for idx in indices:
img_path, _ = dataset.samples[idx]
with Image.open(img_path) as img:
# Convert to grayscale
img_gray = img.convert('L')
pixel_values.extend(list(img_gray.getdata()))
plt.figure(figsize=(10, 4))
plt.hist(pixel_values, bins=50)
plt.title('Pixel Intensity Distribution')
plt.xlabel('Pixel Value')
plt.ylabel('Frequency')
plt.show()
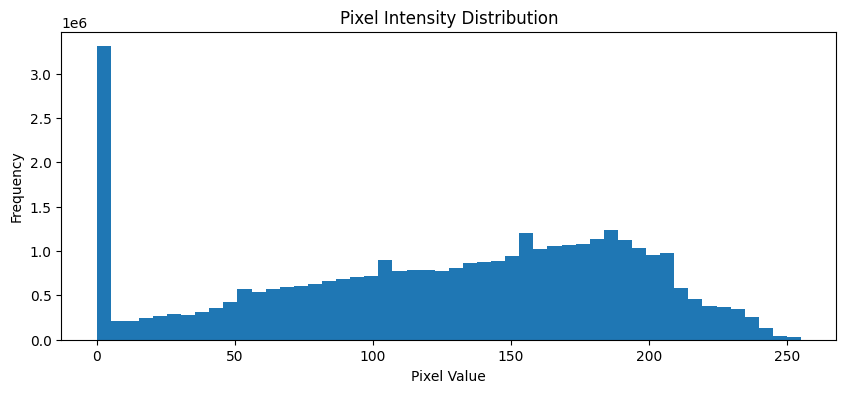
# Analyze pixel distribution
analyze_pixel_distribution(train_dataset)
Data Splitting & Stratification#
One of the most critical aspects of medical image analysis is proper data splitting. Let’s implement it correctly.
⚠️ Common Pitfalls in Medical Image Data Splitting:#
Patient-level splitting: Medical datasets often have multiple images from the same patient. If you split randomly by image, you might have the same patient’s images in both training and validation sets, leading to data leakage.
Class imbalance: Medical datasets typically have imbalanced classes. Use stratified splitting to maintain the same class distribution across splits.
Temporal leakage: In longitudinal studies, images taken at later dates should not be used to predict earlier outcomes.
Let’s implement a patient-level stratified split:
# Create a custom dataset that tracks patient IDs
custom_dataset = ChestXrayDataset(os.path.join(data_dir, "train"), transform=train_transform)
# Get unique patient IDs
patient_ids = list(set([sample[2] for sample in custom_dataset.samples]))
print(f"Total unique patients: {len(patient_ids)}")
# Get labels for each patient (using first image of each patient)
patient_to_label = {}
for path, label, patient_id in custom_dataset.samples:
if patient_id not in patient_to_label:
patient_to_label[patient_id] = label
# Stratified split of patients
from sklearn.model_selection import train_test_split
# Split patient IDs, stratified by the label of each patient
train_patients, val_patients = train_test_split(
patient_ids,
test_size=0.2,
random_state=42,
stratify=[patient_to_label[p] for p in patient_ids]
)
print(f"Training patients: {len(train_patients)}")
print(f"Validation patients: {len(val_patients)}")
# Create new datasets based on the patient split
train_samples = [(path, label) for path, label, patient_id in custom_dataset.samples if patient_id in train_patients]
val_samples = [(path, label) for path, label, patient_id in custom_dataset.samples if patient_id in val_patients]
# Create subset datasets
from torch.utils.data import Subset
class ImageFolderSubset(Dataset):
def __init__(self, samples, transform=None):
self.samples = samples
self.transform = transform
def __len__(self):
return len(self.samples)
def __getitem__(self, idx):
path, label = self.samples[idx]
img = Image.open(path).convert('RGB')
if self.transform:
img = self.transform(img)
return img, label
# Create proper train and validation datasets
proper_train_dataset = ImageFolderSubset(train_samples, transform=train_transform)
proper_val_dataset = ImageFolderSubset(val_samples, transform=val_test_transform)
print(f"Training images: {len(proper_train_dataset)}")
print(f"Validation images: {len(proper_val_dataset)}")
Total unique patients: 2976
Training patients: 2380
Validation patients: 596
Training images: 4166
Validation images: 1050
Creating Data Loaders with Sampling Strategy#
# Create data loaders with appropriate batch size
def create_data_loaders(train_dataset, val_dataset, test_dataset, batch_size=16):
# Calculate weights for weighted sampling to handle class imbalance
train_targets = [label for _, label in train_dataset.samples]
class_counts = np.bincount(train_targets)
class_weights = 1. / torch.tensor(class_counts, dtype=torch.float)
sample_weights = class_weights[train_targets]
# Create weighted sampler
sampler = WeightedRandomSampler(
weights=sample_weights,
num_samples=len(train_dataset),
replacement=True
)
# Create data loaders
train_loader = DataLoader(
train_dataset,
batch_size=batch_size,
sampler=sampler,
num_workers=4,
pin_memory=True
)
val_loader = DataLoader(
val_dataset,
batch_size=batch_size,
shuffle=False,
num_workers=4,
pin_memory=True
)
test_loader = DataLoader(
test_dataset,
batch_size=batch_size,
shuffle=False,
num_workers=4,
pin_memory=True
)
return train_loader, val_loader, test_loader
# Create data loaders
train_loader, val_loader, test_loader = create_data_loaders(
train_dataset,
val_dataset,
test_dataset,
batch_size=16
)
# Display a single batch
def show_batch(dataloader):
for images, labels in dataloader:
fig, axs = plt.subplots(4, 4, figsize=(12, 12))
axs = axs.flatten()
for i in range(16):
if i < len(images):
img = images[i].numpy().transpose((1, 2, 0))
mean = np.array([0.485, 0.456, 0.406])
std = np.array([0.229, 0.224, 0.225])
img = std * img + mean
img = np.clip(img, 0, 1)
axs[i].imshow(img)
axs[i].set_title(f"Label: {train_dataset.classes[labels[i]]}")
axs[i].axis('off')
plt.tight_layout()
plt.show()
break
show_batch(train_loader)
Model Architecture & Transfer Learning#
Transfer learning is particularly important in medical imaging where datasets are often small. Let’s explore how to use pretrained models effectively.
# Define a medical image classification model using transfer learning
class MedicalImageClassifier(nn.Module):
def __init__(self, num_classes=1, model_name="resnet18", pretrained=True, freeze_backbone=False):
super(MedicalImageClassifier, self).__init__()
# Available model architectures
model_dict = {
"resnet18": models.resnet18,
"resnet50": models.resnet50,
"densenet121": models.densenet121,
"efficientnet_b0": models.efficientnet_b0,
"mobilenet_v2": models.mobilenet_v2
}
if model_name not in model_dict:
raise ValueError(f"Model {model_name} not supported")
# Load pretrained backbone
self.backbone = model_dict[model_name](pretrained=pretrained)
# Modify final layer based on architecture
if "resnet" in model_name:
in_features = self.backbone.fc.in_features
self.backbone.fc = nn.Identity()
elif "densenet" in model_name:
in_features = self.backbone.classifier.in_features
self.backbone.classifier = nn.Identity()
elif "efficientnet" in model_name:
in_features = self.backbone.classifier[1].in_features
self.backbone.classifier = nn.Identity()
elif "mobilenet" in model_name:
in_features = self.backbone.classifier[1].in_features
self.backbone.classifier = nn.Identity()
# Freeze backbone parameters if requested
if freeze_backbone:
for param in self.backbone.parameters():
param.requires_grad = False
# Custom classifier head
self.classifier = nn.Sequential(
nn.Dropout(0.2),
nn.Linear(in_features, 256),
nn.ReLU(),
nn.Dropout(0.2),
nn.Linear(256, num_classes)
)
def forward(self, x):
# Extract features
features = self.backbone(x)
# Classify
return self.classifier(features)
# Initialize model
model = MedicalImageClassifier(
num_classes=1, # Binary classification
model_name="resnet18",
pretrained=True,
freeze_backbone=True
)
model = model.to(device)
print(model)
# Summary of model parameters
def count_parameters(model):
return sum(p.numel() for p in model.parameters() if p.requires_grad)
print(f"Number of trainable parameters: {count_parameters(model):,}")
Downloading: "https://download.pytorch.org/models/resnet18-f37072fd.pth" to /root/.cache/torch/hub/checkpoints/resnet18-f37072fd.pth
100%|██████████| 44.7M/44.7M [00:00<00:00, 84.9MB/s]
MedicalImageClassifier(
(backbone): ResNet(
(conv1): Conv2d(3, 64, kernel_size=(7, 7), stride=(2, 2), padding=(3, 3), bias=False)
(bn1): BatchNorm2d(64, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(relu): ReLU(inplace=True)
(maxpool): MaxPool2d(kernel_size=3, stride=2, padding=1, dilation=1, ceil_mode=False)
(layer1): Sequential(
(0): BasicBlock(
(conv1): Conv2d(64, 64, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn1): BatchNorm2d(64, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(relu): ReLU(inplace=True)
(conv2): Conv2d(64, 64, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn2): BatchNorm2d(64, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
)
(1): BasicBlock(
(conv1): Conv2d(64, 64, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn1): BatchNorm2d(64, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(relu): ReLU(inplace=True)
(conv2): Conv2d(64, 64, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn2): BatchNorm2d(64, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
)
)
(layer2): Sequential(
(0): BasicBlock(
(conv1): Conv2d(64, 128, kernel_size=(3, 3), stride=(2, 2), padding=(1, 1), bias=False)
(bn1): BatchNorm2d(128, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(relu): ReLU(inplace=True)
(conv2): Conv2d(128, 128, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn2): BatchNorm2d(128, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(downsample): Sequential(
(0): Conv2d(64, 128, kernel_size=(1, 1), stride=(2, 2), bias=False)
(1): BatchNorm2d(128, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
)
)
(1): BasicBlock(
(conv1): Conv2d(128, 128, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn1): BatchNorm2d(128, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(relu): ReLU(inplace=True)
(conv2): Conv2d(128, 128, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn2): BatchNorm2d(128, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
)
)
(layer3): Sequential(
(0): BasicBlock(
(conv1): Conv2d(128, 256, kernel_size=(3, 3), stride=(2, 2), padding=(1, 1), bias=False)
(bn1): BatchNorm2d(256, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(relu): ReLU(inplace=True)
(conv2): Conv2d(256, 256, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn2): BatchNorm2d(256, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(downsample): Sequential(
(0): Conv2d(128, 256, kernel_size=(1, 1), stride=(2, 2), bias=False)
(1): BatchNorm2d(256, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
)
)
(1): BasicBlock(
(conv1): Conv2d(256, 256, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn1): BatchNorm2d(256, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(relu): ReLU(inplace=True)
(conv2): Conv2d(256, 256, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn2): BatchNorm2d(256, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
)
)
(layer4): Sequential(
(0): BasicBlock(
(conv1): Conv2d(256, 512, kernel_size=(3, 3), stride=(2, 2), padding=(1, 1), bias=False)
(bn1): BatchNorm2d(512, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(relu): ReLU(inplace=True)
(conv2): Conv2d(512, 512, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn2): BatchNorm2d(512, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(downsample): Sequential(
(0): Conv2d(256, 512, kernel_size=(1, 1), stride=(2, 2), bias=False)
(1): BatchNorm2d(512, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
)
)
(1): BasicBlock(
(conv1): Conv2d(512, 512, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn1): BatchNorm2d(512, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
(relu): ReLU(inplace=True)
(conv2): Conv2d(512, 512, kernel_size=(3, 3), stride=(1, 1), padding=(1, 1), bias=False)
(bn2): BatchNorm2d(512, eps=1e-05, momentum=0.1, affine=True, track_running_stats=True)
)
)
(avgpool): AdaptiveAvgPool2d(output_size=(1, 1))
(fc): Identity()
)
(classifier): Sequential(
(0): Dropout(p=0.2, inplace=False)
(1): Linear(in_features=512, out_features=256, bias=True)
(2): ReLU()
(3): Dropout(p=0.2, inplace=False)
(4): Linear(in_features=256, out_features=1, bias=True)
)
)
Number of trainable parameters: 131,585
Loss Function and Optimizer#
# Define loss function (with class weights for imbalance)
class_weights = torch.tensor([1.0, 2.0]).to(device) # Adjust based on your dataset
criterion = nn.BCEWithLogitsLoss()
# Optimizer with weight decay to prevent overfitting
optimizer = optim.Adam(model.parameters(), lr=1e-4, weight_decay=1e-5)
# Learning rate scheduler to reduce LR when model plateaus
scheduler = ReduceLROnPlateau(
optimizer,
mode='min',
factor=0.1,
patience=3,
verbose=True
)
Training with Best Practices#
Let’s implement a robust training loop with validation and early stopping.
def train_one_epoch(model, train_loader, optimizer, criterion, epoch):
model.train()
running_loss = 0.0
# Progress bar
progress = tqdm(enumerate(train_loader), total=len(train_loader),
desc=f"Epoch {epoch+1}/{num_epochs}")
for i, (inputs, labels) in progress:
# Move data to device
inputs = inputs.to(device)
labels = labels.float().unsqueeze(1).to(device)
# Zero gradients
optimizer.zero_grad()
# Forward pass
outputs = model(inputs)
# Calculate loss
loss = criterion(outputs, labels)
# Backward pass and optimize
loss.backward()
optimizer.step()
# Update running loss
running_loss += loss.item()
# Update progress bar
progress.set_postfix({"batch_loss": f"{loss.item():.4f}"})
epoch_loss = running_loss / len(train_loader)
return epoch_loss
def validate(model, val_loader, criterion):
model.eval()
running_loss = 0.0
y_true, y_pred, y_scores = [], [], []
with torch.no_grad():
for inputs, labels in val_loader:
inputs = inputs.to(device)
labels = labels.float().unsqueeze(1).to(device)
# Forward pass
outputs = model(inputs)
# Calculate loss
loss = criterion(outputs, labels)
running_loss += loss.item()
# Store predictions and targets
scores = torch.sigmoid(outputs).cpu().numpy()
preds = (scores > 0.5).astype(int)
y_scores.extend(scores)
y_pred.extend(preds)
y_true.extend(labels.cpu().numpy())
# Calculate metrics
val_loss = running_loss / len(val_loader)
val_acc = accuracy_score(y_true, y_pred)
val_auc = roc_auc_score(y_true, y_scores)
val_f1 = f1_score(y_true, y_pred)
return val_loss, val_acc, val_auc, val_f1, y_true, y_pred, y_scores
# Early stopping
class EarlyStopping:
def __init__(self, patience=7, min_delta=0, verbose=True):
self.patience = patience
self.min_delta = min_delta
self.verbose = verbose
self.counter = 0
self.best_score = None
self.early_stop = False
def __call__(self, val_loss, model):
score = -val_loss
if self.best_score is None:
self.best_score = score
return False
if score < self.best_score + self.min_delta:
self.counter += 1
if self.verbose:
print(f"EarlyStopping counter: {self.counter} out of {self.patience}")
if self.counter >= self.patience:
self.early_stop = True
return True
else:
self.best_score = score
self.counter = 0
return False
Full Training Loop#
# Training settings
from tqdm import tqdm
num_epochs = 5
early_stopping = EarlyStopping(patience=5)
# Track metrics
train_losses = []
val_losses = []
val_accs = []
val_aucs = []
val_f1s = []
# Training loop
for epoch in range(num_epochs):
# Train one epoch
train_loss = train_one_epoch(model, train_loader, optimizer, criterion, epoch)
train_losses.append(train_loss)
# Validate
val_loss, val_acc, val_auc, val_f1, _, _, _ = validate(model, val_loader, criterion)
val_losses.append(val_loss)
val_accs.append(val_acc)
val_aucs.append(val_auc)
val_f1s.append(val_f1)
# Update scheduler
scheduler.step(val_loss)
# Print metrics
print(f"Epoch {epoch+1}/{num_epochs} - "
f"Train Loss: {train_loss:.4f}, "
f"Val Loss: {val_loss:.4f}, "
f"Val Acc: {val_acc:.4f}, "
f"Val AUC: {val_auc:.4f}, "
f"Val F1: {val_f1:.4f}")
# Early stopping
if early_stopping(val_loss, model):
print("Early stopping triggered")
break
# Save the best model
torch.save(model.state_dict(), "best_model.pth")
# Plot training history
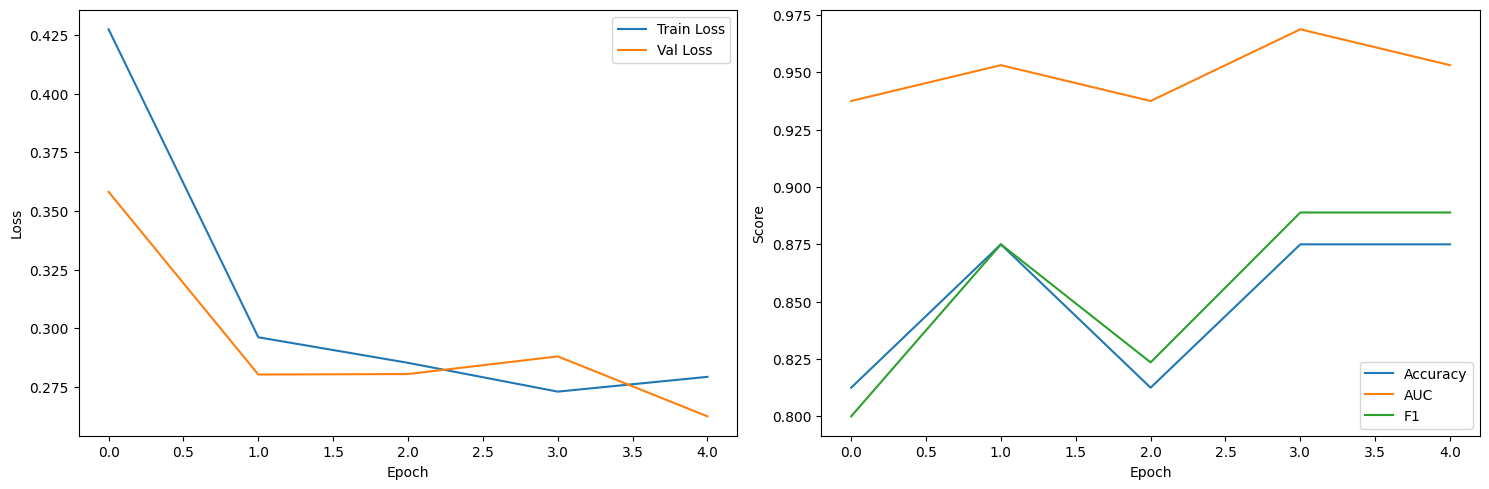
plt.figure(figsize=(15, 5))
plt.subplot(1, 2, 1)
plt.plot(train_losses, label='Train Loss')
plt.plot(val_losses, label='Val Loss')
plt.xlabel('Epoch')
plt.ylabel('Loss')
plt.legend()
plt.subplot(1, 2, 2)
plt.plot(val_accs, label='Accuracy')
plt.plot(val_aucs, label='AUC')
plt.plot(val_f1s, label='F1')
plt.xlabel('Epoch')
plt.ylabel('Score')
plt.legend()
plt.tight_layout()
plt.show()
Epoch 1/5: 100%|██████████| 326/326 [02:14<00:00, 2.42it/s, batch_loss=0.2947]
Epoch 1/5 - Train Loss: 0.4273, Val Loss: 0.3581, Val Acc: 0.8125, Val AUC: 0.9375, Val F1: 0.8000
Epoch 2/5: 100%|██████████| 326/326 [02:15<00:00, 2.41it/s, batch_loss=0.2092]
Epoch 2/5 - Train Loss: 0.2962, Val Loss: 0.2804, Val Acc: 0.8750, Val AUC: 0.9531, Val F1: 0.8750
Epoch 3/5: 100%|██████████| 326/326 [02:15<00:00, 2.41it/s, batch_loss=0.3906]
Epoch 3/5 - Train Loss: 0.2853, Val Loss: 0.2806, Val Acc: 0.8125, Val AUC: 0.9375, Val F1: 0.8235
EarlyStopping counter: 1 out of 5
Epoch 4/5: 100%|██████████| 326/326 [02:14<00:00, 2.42it/s, batch_loss=0.2494]
Epoch 4/5 - Train Loss: 0.2731, Val Loss: 0.2881, Val Acc: 0.8750, Val AUC: 0.9688, Val F1: 0.8889
EarlyStopping counter: 2 out of 5
Epoch 5/5: 100%|██████████| 326/326 [02:20<00:00, 2.32it/s, batch_loss=0.2693]
Epoch 5/5 - Train Loss: 0.2794, Val Loss: 0.2626, Val Acc: 0.8750, Val AUC: 0.9531, Val F1: 0.8889
Advanced Evaluation Metrics#
For medical applications, it’s crucial to go beyond accuracy. Let’s implement comprehensive evaluation metrics.
def evaluate_model_comprehensively(model, test_loader):
# Set model to evaluation mode
model.eval()
# Lists to store predictions and targets
y_true, y_pred, y_scores = [], [], []
# Disable gradient calculations
with torch.no_grad():
for inputs, labels in test_loader:
inputs = inputs.to(device)
# Forward pass
outputs = model(inputs)
probs = torch.sigmoid(outputs).cpu().squeeze().numpy()
preds = (probs > 0.5).astype(int)
# Store predictions and targets
y_scores.extend(probs if isinstance(probs, np.ndarray) else [probs])
y_pred.extend(preds if isinstance(preds, np.ndarray) else [preds])
y_true.extend(labels.cpu().numpy())
# Convert to numpy arrays for easier manipulation
y_true = np.array(y_true)
y_pred = np.array(y_pred)
y_scores = np.array(y_scores)
# Calculate metrics
print("\n===== Model Performance =====")
print(classification_report(y_true, y_pred))
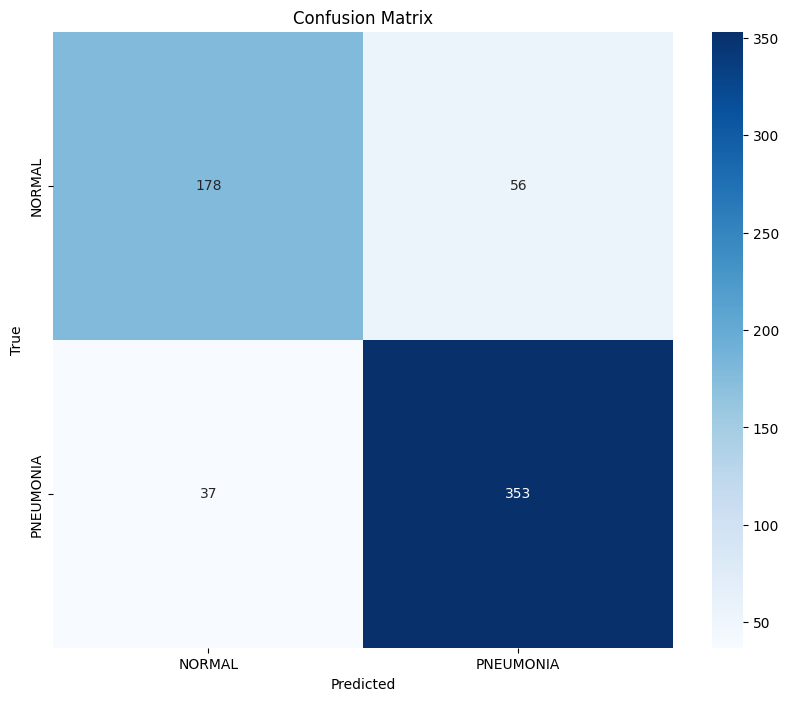
# Calculate and plot confusion matrix
cm = confusion_matrix(y_true, y_pred)
plt.figure(figsize=(10, 8))
sns.heatmap(cm, annot=True, fmt='d', cmap='Blues',
xticklabels=test_dataset.classes,
yticklabels=test_dataset.classes)
plt.xlabel('Predicted')
plt.ylabel('True')
plt.title('Confusion Matrix')
plt.show()
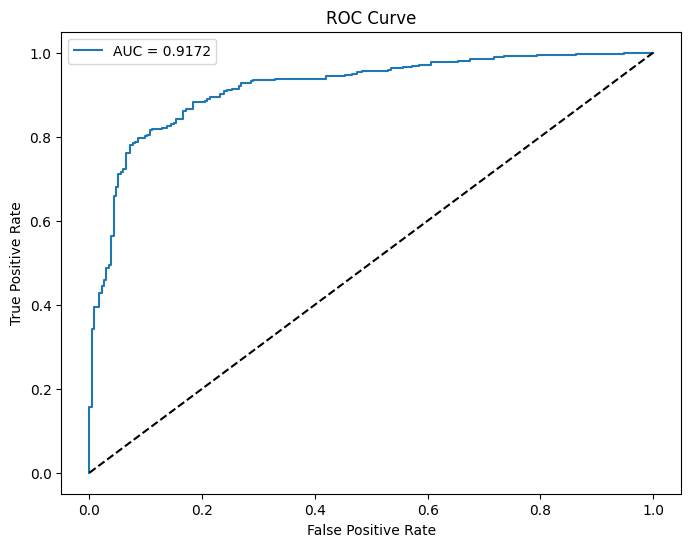
# Calculate AUC-ROC
auc_roc = roc_auc_score(y_true, y_scores)
print(f"AUC-ROC: {auc_roc:.4f}")
# Plot ROC curve
fpr, tpr, _ = roc_curve(y_true, y_scores)
plt.figure(figsize=(8, 6))
plt.plot(fpr, tpr, label=f'AUC = {auc_roc:.4f}')
plt.plot([0, 1], [0, 1], 'k--')
plt.xlabel('False Positive Rate')
plt.ylabel('True Positive Rate')
plt.title('ROC Curve')
plt.legend()
plt.show()
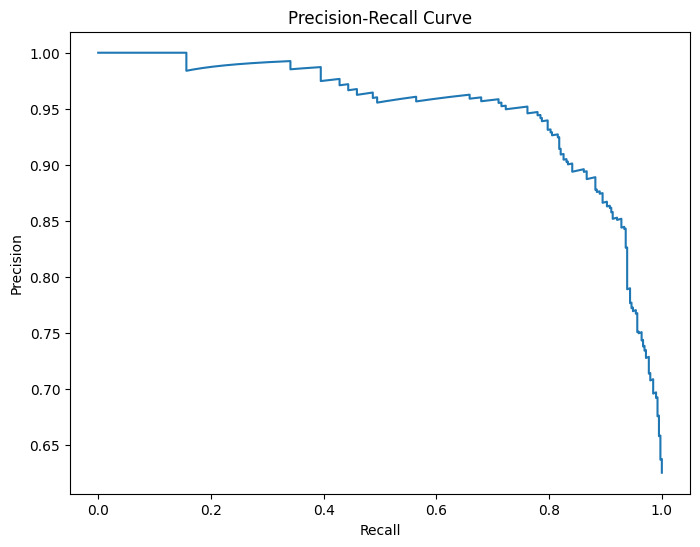
# Plot precision-recall curve
precision, recall, _ = precision_recall_curve(y_true, y_scores)
plt.figure(figsize=(8, 6))
plt.plot(recall, precision)
plt.xlabel('Recall')
plt.ylabel('Precision')
plt.title('Precision-Recall Curve')
plt.show()
# Calculate F1 score at different thresholds
thresholds = np.arange(0.1, 1.0, 0.1)
f1_scores = []
for thresh in thresholds:
thresh_preds = (y_scores > thresh).astype(int)
f1 = f1_score(y_true, thresh_preds)
f1_scores.append(f1)
# Plot F1 scores at different thresholds
plt.figure(figsize=(8, 6))
plt.plot(thresholds, f1_scores, marker='o')
plt.xlabel('Threshold')
plt.ylabel('F1 Score')
plt.title('F1 Score vs Threshold')
plt.grid(True)
plt.show()
# Find optimal threshold
best_threshold_idx = np.argmax(f1_scores)
best_threshold = thresholds[best_threshold_idx]
print(f"Optimal threshold: {best_threshold:.2f} with F1 score: {f1_scores[best_threshold_idx]:.4f}")
return y_true, y_pred, y_scores
# Evaluate the model
y_true, y_pred, y_scores = evaluate_model_comprehensively(model, test_loader)
===== Model Performance =====
precision recall f1-score support
0 0.83 0.76 0.79 234
1 0.86 0.91 0.88 390
accuracy 0.85 624
macro avg 0.85 0.83 0.84 624
weighted avg 0.85 0.85 0.85 624
AUC-ROC: 0.9172
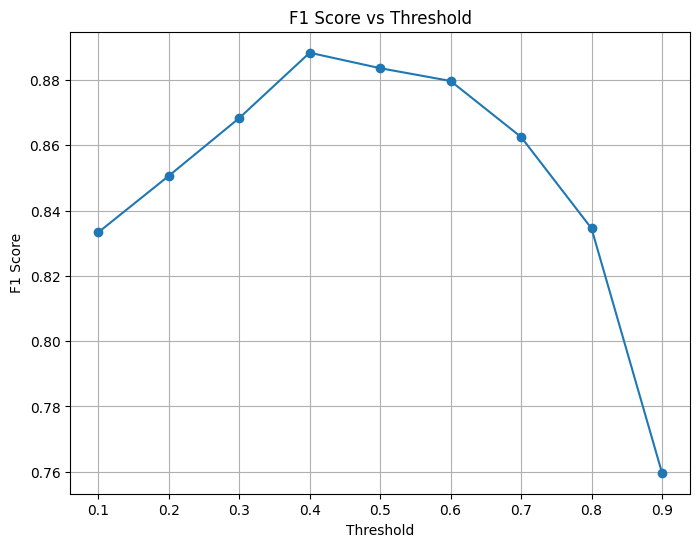
Optimal threshold: 0.40 with F1 score: 0.8883
Handling Class Imbalance#
Medical datasets often suffer from class imbalance. Let’s explore techniques to address this issue.

def analyze_class_imbalance(train_dataset, val_dataset, test_dataset):
"""Analyze class imbalance in the datasets"""
# Count samples per class
train_counts = count_samples_per_class(train_dataset)
val_counts = count_samples_per_class(val_dataset)
test_counts = count_samples_per_class(test_dataset)
# Plot class distribution
labels = list(train_counts.keys())
train_values = [train_counts[label] for label in labels]
val_values = [val_counts[label] for label in labels]
test_values = [test_counts[label] for label in labels]
x = np.arange(len(labels))
width = 0.25
fig, ax = plt.subplots(figsize=(10, 6))
ax.bar(x - width, train_values, width, label='Train')
ax.bar(x, val_values, width, label='Validation')
ax.bar(x + width, test_values, width, label='Test')
ax.set_ylabel('Number of samples')
ax.set_title('Class distribution across datasets')
ax.set_xticks(x)
ax.set_xticklabels(labels)
ax.legend()
plt.tight_layout()
plt.show()
# Calculate imbalance ratio
train_ratio = max(train_values) / min(train_values)
print(f"Imbalance ratio in training set: {train_ratio:.2f}:1")
# Suggest mitigation strategies
print("\n===== Class Imbalance Mitigation Strategies =====")
if train_ratio > 10:
imbalance_level = "Severe"
elif train_ratio > 3:
imbalance_level = "Moderate"
else:
imbalance_level = "Mild"
print(f"Level of imbalance: {imbalance_level}")
print("\nRecommended strategies:")
strategies = [
"Data augmentation for minority classes",
"Class weighting in loss function",
"Oversampling minority classes",
"Undersampling majority classes",
"Generation of synthetic samples (SMOTE)",
"Ensemble methods with balanced sub-datasets",
"Focal Loss to focus on hard examples"
]
for strategy in strategies:
print(f"- {strategy}")
# Analyze class imbalance
analyze_class_imbalance(train_dataset, val_dataset, test_dataset)
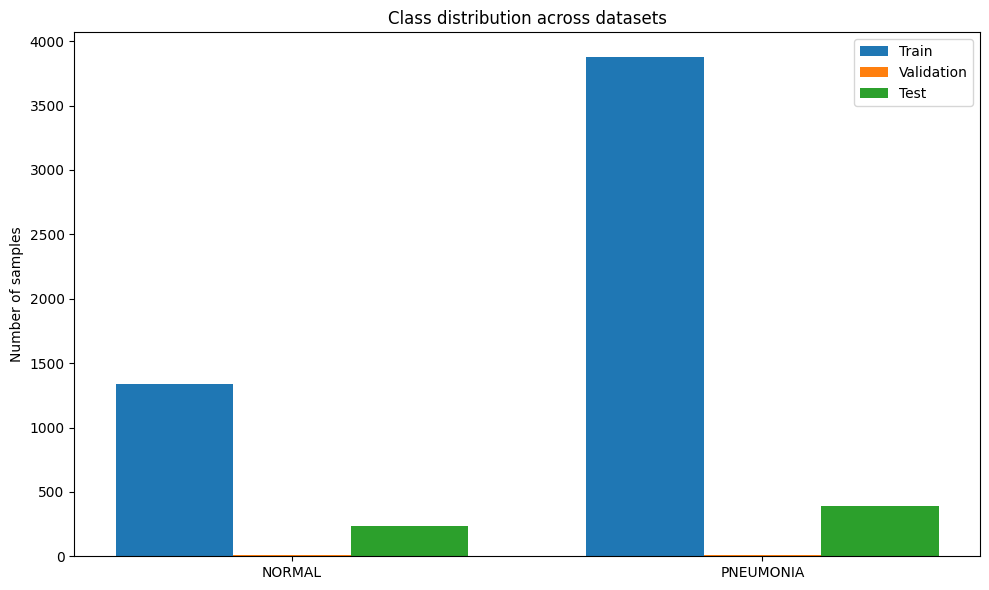
Imbalance ratio in training set: 2.89:1
===== Class Imbalance Mitigation Strategies =====
Level of imbalance: Mild
Recommended strategies:
- Data augmentation for minority classes
- Class weighting in loss function
- Oversampling minority classes
- Undersampling majority classes
- Generation of synthetic samples (SMOTE)
- Ensemble methods with balanced sub-datasets
- Focal Loss to focus on hard examples
Implement Class Weighting#
def implement_class_weighting():
"""Implement weighted loss function for imbalanced classes"""
# Calculate class weights inversely proportional to frequency
train_targets = [label for _, label in train_dataset.samples]
class_counts = np.bincount(train_targets)
n_samples = len(train_targets)
class_weights = n_samples / (len(class_counts) * class_counts)
class_weights = torch.FloatTensor(class_weights).to(device)
print(f"Class weights: {class_weights.cpu().numpy()}")
# Define weighted loss function
weighted_criterion = nn.BCEWithLogitsLoss(pos_weight=class_weights[1])
return weighted_criterion
# Create weighted loss function
weighted_criterion = implement_class_weighting()
Class weights: [1.9448173 0.6730323]
Implement Balanced Sampling#
def create_balanced_sampler(dataset):
"""Create a balanced sampler for imbalanced datasets"""
targets = [label for _, label in dataset.samples]
# Calculate class weights inversely proportional to frequency
class_counts = np.bincount(targets)
class_weights = 1. / torch.tensor(class_counts, dtype=torch.float)
# Assign weight to each sample
sample_weights = class_weights[targets]
# Create weighted sampler
sampler = WeightedRandomSampler(
weights=sample_weights,
num_samples=len(dataset),
replacement=True
)
return sampler
# Create balanced sampler for training
balanced_sampler = create_balanced_sampler(train_dataset)
# Create data loader with balanced sampler
balanced_train_loader = DataLoader(
train_dataset,
batch_size=16,
sampler=balanced_sampler,
num_workers=4,
pin_memory=True
)
Cross-Validation Strategies#
For medical image classifiers, robust validation is essential. Let’s implement k-fold cross-validation with stratification.

def perform_cross_validation(model_class, dataset, num_folds=5, num_epochs=10):
"""Perform k-fold cross-validation"""
# Get labels for stratification
labels = [label for _, label in dataset.samples]
# Create stratified k-fold splitter
skf = StratifiedKFold(n_splits=num_folds, shuffle=True, random_state=42)
# Track metrics for each fold
fold_metrics = {
'accuracy': [],
'auc': [],
'f1': [],
'precision': [],
'recall': []
}
# Perform k-fold cross-validation
for fold, (train_idx, val_idx) in enumerate(skf.split(np.zeros(len(labels)), labels)):
print(f"\n===== Fold {fold+1}/{num_folds} =====")
# Create datasets for this fold
train_subset = Subset(dataset, train_idx)
val_subset = Subset(dataset, val_idx)
# Create data loaders
train_loader = DataLoader(train_subset, batch_size=16, shuffle=True, num_workers=4)
val_loader = DataLoader(val_subset, batch_size=16, shuffle=False, num_workers=4)
# Initialize model
fold_model = model_class(
num_classes=1,
model_name="resnet18",
pretrained=True,
freeze_backbone=False
).to(device)
# Initialize optimizer and loss
optimizer = optim.Adam(fold_model.parameters(), lr=1e-4, weight_decay=1e-5)
criterion = nn.BCEWithLogitsLoss()
# Train the model for this fold
for epoch in range(num_epochs):
# Train one epoch
fold_model.train()
for inputs, labels in train_loader:
inputs = inputs.to(device)
labels = labels.float().unsqueeze(1).to(device)
optimizer.zero_grad()
outputs = fold_model(inputs)
loss = criterion(outputs, labels)
loss.backward()
optimizer.step()
# Validate
val_loss, val_acc, val_auc, val_f1, y_true, y_pred, _ = validate(fold_model, val_loader, criterion)
print(f"Epoch {epoch+1}/{num_epochs} - "
f"Val Loss: {val_loss:.4f}, "
f"Val Acc: {val_acc:.4f}, "
f"Val AUC: {val_auc:.4f}, "
f"Val F1: {val_f1:.4f}")
# Final evaluation for this fold
fold_model.eval()
y_true, y_pred, y_scores = [], [], []
with torch.no_grad():
for inputs, labels in val_loader:
inputs = inputs.to(device)
outputs = fold_model(inputs)
probs = torch.sigmoid(outputs).cpu().numpy()
preds = (probs > 0.5).astype(int)
y_scores.extend(probs)
y_pred.extend(preds)
y_true.extend(labels.numpy())
# Calculate metrics for this fold
fold_metrics['accuracy'].append(accuracy_score(y_true, y_pred))
fold_metrics['auc'].append(roc_auc_score(y_true, y_scores))
fold_metrics['f1'].append(f1_score(y_true, y_pred))
fold_metrics['precision'].append(precision_score(y_true, y_pred))
fold_metrics['recall'].append(recall_score(y_true, y_pred))
# Print summary of cross-validation
print("\n===== Cross-Validation Results =====")
for metric, scores in fold_metrics.items():
print(f"{metric}: {np.mean(scores):.4f} ± {np.std(scores):.4f}")
return fold_metrics
# Perform cross-validation
cv_metrics = perform_cross_validation(MedicalImageClassifier, train_dataset, num_folds=3, num_epochs=5)
===== Fold 1/3 =====
Epoch 1/5 - Val Loss: 0.0657, Val Acc: 0.9764, Val AUC: 0.9977, Val F1: 0.9840
Epoch 2/5 - Val Loss: 0.1008, Val Acc: 0.9661, Val AUC: 0.9972, Val F1: 0.9777
Epoch 3/5 - Val Loss: 0.0530, Val Acc: 0.9799, Val AUC: 0.9979, Val F1: 0.9864
Epoch 4/5 - Val Loss: 0.0415, Val Acc: 0.9856, Val AUC: 0.9982, Val F1: 0.9903
Epoch 5/5 - Val Loss: 0.0597, Val Acc: 0.9770, Val AUC: 0.9984, Val F1: 0.9847
===== Fold 2/3 =====
Epoch 1/5 - Val Loss: 0.0830, Val Acc: 0.9672, Val AUC: 0.9956, Val F1: 0.9777
Epoch 2/5 - Val Loss: 0.1243, Val Acc: 0.9592, Val AUC: 0.9899, Val F1: 0.9731
Epoch 3/5 - Val Loss: 0.0499, Val Acc: 0.9810, Val AUC: 0.9981, Val F1: 0.9873
Epoch 4/5 - Val Loss: 0.2047, Val Acc: 0.9310, Val AUC: 0.9915, Val F1: 0.9556
Epoch 5/5 - Val Loss: 0.0856, Val Acc: 0.9666, Val AUC: 0.9979, Val F1: 0.9771
===== Fold 3/3 =====
Epoch 1/5 - Val Loss: 0.0588, Val Acc: 0.9816, Val AUC: 0.9970, Val F1: 0.9876
Epoch 2/5 - Val Loss: 0.0582, Val Acc: 0.9793, Val AUC: 0.9964, Val F1: 0.9861
Epoch 3/5 - Val Loss: 0.0541, Val Acc: 0.9758, Val AUC: 0.9980, Val F1: 0.9839
Epoch 4/5 - Val Loss: 0.0618, Val Acc: 0.9735, Val AUC: 0.9974, Val F1: 0.9823
Epoch 5/5 - Val Loss: 0.0348, Val Acc: 0.9850, Val AUC: 0.9991, Val F1: 0.9899
===== Cross-Validation Results =====
accuracy: 0.9739 ± 0.0085
auc: 0.9984 ± 0.0005
f1: 0.9824 ± 0.0059
precision: 0.9835 ± 0.0106
recall: 0.9817 ± 0.0178
Summary and Next Steps#
Recap of the session#
🗂️ Dataset Loading and Inspection#
✅ DOs
Load image data in the correct format (e.g., DICOM, PNG, NIfTI).
Validate image dimensions and channel configuration.
Map patient IDs to image files to avoid data leakage.
Visualize random samples from each class for sanity check.
Inspect and understand metadata fields before dropping them.
❌ DON’Ts
Don’t assume all images are the same shape or format.
Don’t ignore metadata—it may contain patient info or label cues.
Don’t load entire datasets into memory if they’re too large.
Don’t blindly trust the labels in public datasets without validation.
🔬 Exploratory Data Analysis & Preprocessing#
✅ DOs
Plot class distribution to detect imbalance.
Visualize pixel intensity histograms before normalization.
Apply medically-valid augmentations (rotation, crop, brightness).
Normalize images consistently across train/val/test.
Save and document preprocessing steps for reproducibility.
❌ DON’Ts
Don’t apply random left-right flips unless medically valid.
Don’t normalize before inspecting image characteristics.
Don’t augment validation or test datasets.
Don’t lose track of image-patient relationships.
✂️ Data Splitting & Stratification#
✅ DOs
Split data based on patient ID, not images.
Stratify splits to preserve class distributions.
Set aside a fixed test set early and keep it untouched.
Use tools like
train_test_splitwith stratification for reproducibility.
❌ DON’Ts
Don’t perform random image-level splitting.
Don’t peek into the test set during model development.
Don’t reuse patients across training and validation.
Don’t overlook multi-label stratification complexity.
🧱 Model Architecture & Transfer Learning#
✅ DOs
Start with a simple pretrained model (e.g., ResNet18).
Adapt the final classification head to match your task.
Match input channels to your image format (e.g., 1 for grayscale).
Freeze base layers initially, then fine-tune gradually.
❌ DON’Ts
Don’t use overly complex models for small datasets.
Don’t forget to switch from softmax to sigmoid for multi-label tasks.
Don’t ignore mismatched input shapes or channels.
Don’t assume ImageNet pretraining is always optimal for medical data.
🏋️ Training with Best Practices#
✅ DOs
Monitor training/validation loss and key metrics regularly.
Use callbacks like early stopping and checkpointing.
Tune learning rate using a scheduler.
Enable mixed precision training for speed if available.
❌ DON’Ts
Don’t rely on a single metric like accuracy.
Don’t overfit on the training data.
Don’t ignore convergence issues (e.g., exploding/vanishing gradients).
Don’t train without fixing random seeds if you want reproducibility.
📊 Advanced Evaluation Metrics#
✅ DOs
Use precision, recall, and F1-score to capture performance.
Visualize ROC and PR curves per class.
Report AUC scores for both ROC and PR when imbalance exists.
Generate confusion matrices and class-wise breakdowns.
❌ DON’Ts
Don’t rely on accuracy, especially with imbalanced datasets.
Don’t evaluate using validation data post-hoc—use a test set.
Don’t ignore class-specific performance in multi-label tasks.
Don’t skip metric analysis across multiple thresholds.
⚖️ Handling Class Imbalance#
✅ DOs
Use class weights or weighted loss functions.
Consider focal loss to reduce bias on majority classes.
Apply augmentations selectively on minority classes.
Track class-wise performance metrics during training.
❌ DON’Ts
Don’t oversample without validation—it can cause overfitting.
Don’t rely on accuracy to claim performance on minority classes.
Don’t use SMOTE or synthetic sampling unless medically reviewed.
Don’t ignore imbalance just because AUC is high.
🔁 Cross-Validation Strategies#
✅ DOs
Use k-fold cross-validation based on patient IDs.
Ensure label distribution is preserved across folds.
Average metrics across folds for robust evaluation.
Use nested CV if hyperparameter tuning is involved.
❌ DON’Ts
Don’t use image-level splitting in CV.
Don’t leak validation data into training folds.
Don’t report metrics from only the best-performing fold.
Don’t ignore variability between folds—report mean and std.
Conclusion#
Developing robust medical image classifiers requires attention to detail at every step of the process, from data preparation to deployment. By following the best practices outlined in this tutorial and avoiding common pitfalls, you can build models that are not only technically sound but also clinically relevant and robust enough for real-world healthcare applications.
Remember that medical AI development should always be done in collaboration with healthcare professionals to ensure that the models address genuine clinical needs and are integrated into healthcare workflows appropriately.
Thank you for following this comprehensive tutorial. We hope it helps you develop high-quality medical image classifiers that can make a positive impact in healthcare.
References and Further Reading#
Rajpurkar, P., et al. (2017). CheXNet: Radiologist-Level Pneumonia Detection on Chest X-Rays with Deep Learning. arXiv preprint arXiv:1711.05225.
Gulshan, V., et al. (2016). Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA, 316(22), 2402-2410.
Cohen, J.P., et al. (2020). COVID-19 Image Data Collection: Prospective Predictions Are the Future. arXiv preprint arXiv:2006.11988.
Selvaraju, R.R., et al. (2017). Grad-CAM: Visual Explanations from Deep Networks via Gradient-based Localization. ICCV 2017.
Yala, A., et al. (2019). A Deep Learning Mammography-based Model for Improved Breast Cancer Risk Prediction. Radiology, 292(1), 60-66.
Kelly, C.J., et al. (2019). Key challenges for delivering clinical impact with artificial intelligence. BMC Medicine, 17(1), 195.
List of the question that asked during the tutorials#
What are the way to determines the if there is a problem with our dataset without plotting ?
Should we apply data data propressing in the data loader or before loading the data ?
How to know if the data augmentation will damage the medical image inside the image ?
